
Contact
Reports
Anti-counterfeiting
Zenosim
home
ZENOSIM 10IU is a sterile, non-pyrogenic, white, lyophilized powder intended for subcutaneous or intramuscular injection after reconstitution with Bacteriostatic Water for Injection, USP (0.9% Benzyl Alcohol).
The reconstituted solution has a pH of 6.5 to 10 ZENOSIM 10IU is available in 10IU somatropin vial composition. Somatropin is a form of human growth hormone. Human growth hormone is important in the body for the growth of bones and muscles.
Somatropin is also used to prevent severe weight loss in people with AIDS, or to treat short bowel syndromeand may also be used for other purposes not listed in this medication guide.
Before you receive somatropin, tell your doctor about all your past and present medical conditions, especially allergies, trauma, surgery, diabetes, cancer, breathing problems, liver or kidney disease, scoliosis, high blood pressure, pancreas disorder, underactive thyroid, or a brain tumor.
Also tell your doctor about all other medications you use, especially steroids or diabetes medications. Your dosages of these medicines may need to be changed when you start using somatropin. Do not stop using a steroid suddenly or change any of your medication doses without your doctor’s advice

ZENOSIM 10IU treats the following conditions:
Growth Hormone Deficiency
Hypopituitarism in Adults
Turner Syndrome
Idiopathic Short Stature
Short stature childhood cases
Somatropin is used to treat growth failure in children and adults who lack natural growth hormone, and in those with chronic kidney failure, Noonan syndrome, Turner syndrome, short stature at birth with no catch-up growth, and other causes.
Before you receive somatropin, tell your doctor if you have ever had an allergic reaction to a growth hormone medicine, or to drug preservatives such as benzyl alcohol, metacresol or glycerin.
You should not use this medication if you are allergic to somatropin, or if you have:diabetic retinopathy (a serious eye condition caused by diabetes), cancer or Prader-Willi syndrome and are also overweight or have sleep apnea or severe respiratory (lung) problems.
You should also not use somatropin if you have a serious medical condition after having: open heart surgery or stomach surgery; trauma or other medical emergency; or breathing problems (such as lung failure).
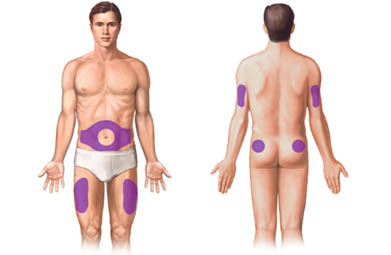
Where to inject ZENOSIM 10IU:
Below are presented the spots where you can inject ZENOSIM 10IU by subcutaneous injection.
How to injection: either subcutaneous or intramuscular. Not Intradermal.
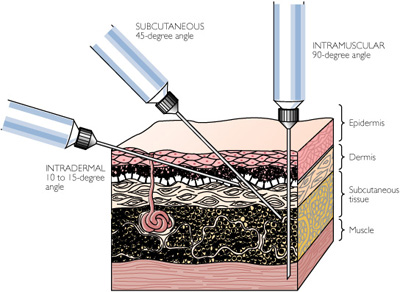
How to injection: either subcutaneous or intramuscular. Not Intradermal.Adverse reactions:
Growth Hormone Deficient Pediatric Patients
As with all protein pharmaceuticals, a small percentage of patients may develop antibodies to the protein. Anti-growth hormone (GH) antibody capacities below 2 mg/L have not been associated with growth attenuation. In some cases when binding capacity exceeds 2 mg/L, growth attenuation has been described. In clinical studies with ZENOSIM 10IU involving 280 patients (204 naive and 76 transfer patients), one patient at 6 months of therapy developed anti-GH antibodies with binding capacities exceeding 2 mg/L. Despite the high binding capacity, these antibodies were not growth attenuating. The patient was subsequently shown to have a hGH-N gene defect.Thus, genetic analysis should be undertaken in any patient in whom anti-GH antibodies with high binding capacities occur.
No antibodies against proteins of the host cells were detected in the sera of patients treated up to five years. Any patient with well-documented growth hormone deficiency who fails to respond to therapy should be tested for antibodies to human growth hormone and for thyroid status. In clinical studies in which ZENOSIM 10IU was administered to growth hormone deficient children, the following events were infrequently seen: local reactions at the injection site (such as pain, numbness, redness and swelling), hypothyroidism, hypoglycemia, seizures, exacerbation of preexisting psoriasis and disturbances in fluid balance. Leukemia has been reported in a small number of growth hormone deficient patients treated with growth hormone.
It is uncertain whether this increased risk is related to the pathology of growth hormone deficiency itself, growth hormone therapy, or other associated treatments such as radiation therapy for intracranial tumors. So far, epidemiological data fail to confirm the hypothesis of a relationship between growth hormone therapy and leukemia.
Growth Hormone Deficient Adult Patients
During the 6 month placebo-controlled study, adverse events were reported in 56 patients (93.3%) in the somatropin-treated group and 42 patients (76.4%) in the placebo-treated group. Arthralgia, myalgia, peripheral edema, other types of edema, carpal tunnel syndrome, paraesthesia and hypoaesthesia were common in the somatropin-treated patients and reported more frequently than in the placebo group.
These types of adverse events are thought to be related to the fluid accumulating effects of somatropin. During the placebo-controlled portion of the study, approximately 10% of patients without preexisting diabetes mellitus or impaired glucose tolerance treated with somatropin manifested mild, but persistent, abnormalities of glucose tolerance, compared with none in the placebo group. During the open label phase of the study, approximately 10% of patients treated with somatropin required a small upward adjustment of thyroid hormone replacement therapy for preexisting central hypothyroidism and 1 patient was newly diagnosed with central hypothyroidism.
In addition, during the open label phase of the study, when all patients were being treated with somatropin, two patients with preexisting central hypoadrenalism required upward titration of hydrocortisone maintenance therapy which was considered to be suboptimal (unrelated to intercurrent stress, surgery or disease), and 1 patient was diagnosed de novo with central adrenal insufficiency after six months of somatropin treatment. Anti-GH antibodies were not detected.
OVERDOSAGE: Short-term overdosage could lead initially to hypoglycemia and subsequently to hyperglycemia. Moreover, overdose with somatropin is likely to cause fluid retention. Long-term overdosage could result in signs and symptoms of gigantism and/or acromegaly consistent with the known effects of excess human growth hormone.
Somatropin inhibits 11β-hydroxysteroid dehydrogenase type 1 (11βHSD-1) in adipose/hepatic tissue and may significantly impact the metabolism of cortisol and cortisone. As a consequence, in patients treated with somatropin, previously undiagnosed central (secondary) hypoadrenalism may be unmasked requiring glucocorticoid replacement therapy. In addition, patients treated with glucocorticoid replacement therapy for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses; this may be especially true for patients treated with cortisone acetate and prednisone since conversion of these drugs to their biologically active metabolites is dependent on the activity of the 11βHSD-1 enzyme.
Growth hormone deficiency:
Short called GHD
aGHD (adult GHD)
Growth hormone deficiency occurs when the pituitary gland – which secretes hormones regulating homeostasis– produces an low amount of growth hormone. This deficiency in growth hormone results in a slower rate of growth.
What causes growth hormone deficiency?
There are a number of possible causes for poor growth hormone production in childhood. There may have been improper formation of the hypothalamus (one of the most important functions of the hypothalamus is to link the nervous system to the endocrine system via the pituitary gland (hypophysis) or pituitary gland itself before birth.
Head injuries, brain tumors, and brain damage due to disease or iradiation can also cause poor functioning of the pituitary gland. There are various ways to determine GHD including stim-tests.
Treatment of growth hormone deficiency
ZENOSIM 10IU is used for the treatment of children who have growth failure due to low production of natural growth hormone but also in adults that suffer of aGHD (adult Growth Hormone Deficiency).
Reconstitution of ZENOSIM 10IU:
Vial — Each 10IU vial of ZENOSIM 10IU should be reconstituted with 1 to 2 ml of Bacteriostatic Water for Injection. The diluent should be injected into the vial of ZENOSIM 10IU by aiming the stream of liquid against the glass wall. Following reconstitution, the vial should be swirled with a GENTLE rotary motion until the contents are completely dissolved. DO NOT SHAKE. The resulting solution should be inspected for clarity. It should be clear. If the solution is cloudy or
contains particulate matter, the contents MUST NOT be injected. Before and after injection, the septum of the vial should be wiped with rubbing alcohol or an
alcoholic antiseptic solution to prevent contamination of the contents by repeated needle
insertions.
Contraindications:
ZENOSIM 10IU is contraindicated in patients with a known hypersensitivity to somatropin or any of its excipients. ZENOSIM 10IU reconstituted with Bacteriostatic Water for Injection, USP (0.9% Benzyl Alcohol) should not be administered to patients with a known sensitivity to Benzyl Alcohol (see Warnings). Somatropin should not be used for growth promotion in pediatric patients with closed epiphyses. Somatropin is contraindicated in patients with active proliferative or severe non-proliferative diabetic retinopathy. In general, somatropin is contraindicated in the presence of active malignancy. Any pre-existing malignancy should be inactive and its treatment complete prior to instituting therapy with somatropin. Somatropin should be discontinued if there is evidence of recurrent activity.
Since growth hormone deficiency may be an early sign of the presence of a pituitary tumor (or, rarely, other brain tumors), the presence of such tumors should be ruled out prior to initiation of treatment. Somatropin should not be used in patients with any evidence of progression or recurrence of an underlying intracranial tumor.Somatropin should not be used to treat patients with acute critical illness due to complications following open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure. Somatropin is contraindicated in patients with Prader-Willi syndrome who are severely obese or have severe respiratory impairment unless patients with Prader-Willi syndrome also have a diagnosis of growth hormone deficiency.
Drug interactions:
Somatropin inhibits 11β-hydroxysteroid dehydrogenase type 1 (11βHSD-1) in adipose/hepatic tissue and may significantly impact the metabolism of cortisol and cortisone. As a consequence, in patients treated with somatropin, previously undiagnosed central (secondary) hypoadrenalism may be unmasked requiring glucocorticoid replacement therapy. In addition, patients treated with glucocorticoid replacement therapy for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses; this may be especially true for patients treated with cortisone acetate and prednisone since conversion of these drugs to their biologically active metabolites is dependent on the activity of the 11βHSD-1 enzyme.
Excessive glucocorticoid therapy may attenuate the growth promoting effects of somatropin in children. Therefore, glucocorticoid replacement therapy should be carefully adjusted in children with concomitant GH and glucocorticoid deficiency to avoid both hypoadrenalism and an inhibitory effect on growth. Limited published data indicate that somatropin treatment increases cytochrome P450 (CP450) mediated antipyrine clearance in man. These data suggest that somatropin administration may alter the clearance of compounds known to be metabolized by CP450 liver enzymes (e.g., corticosteroids, sex steroids, anticonvulsants, cyclosporin). Careful monitoring is advisable when somatropin is administered in combination with other drugs known to be metabolized by CP450 liver enzymes. However, formal drug interaction studies have not been conducted.
In adult women on oral estrogen replacement, a larger dose of somatropin may be required to achieve the defined treatment goal (see DOSAGE AND ADMINISTRATION).
In patients with diabetes mellitus requiring drug therapy, the dose of insulin and/or oral agent may require adjustment when somatropin therapy is initiated

Some medicines given by injection may sometimes be given at home to patients who do not need to be in the hospital. If you are using this medicine at home, your health care professional will teach you how to prepare and inject the medicine. You will have a chance to practice preparing and injecting it. Be certain that you understand exactly how the medicine is to be prepared and injected.
It is important to read the patient information and instructions for use, if provided with your medicine, each time your prescription is filled.
It is important to follow any instructions from your doctor about the careful selection and rotation of injection sites on your body . This will help to prevent skin problems.
If you have certain conditions, you may need a dose adjustment or special tests to safely use this medication. Before using somatropin, tell your doctor if you have: liver disease; kidney disease (or if you are on dialysis); diabetes; scoliosis; high blood pressure (hypertension); a pancreas disorder; a history of cancer; carpal tunnel syndrome; underactive thyroid; or a brain tumor or lesion. FDA pregnancy category C. It is not known whether somatropin is harmful to an unborn baby. Before using this medication, tell your doctor if you are pregnant or plan to become pregnant during treatment. It is not known whether somatropin passes into breast milk or if it could harm a nursing baby. Do not use somatropin without telling your doctor if you are breast-feeding a baby.
Dosing ZENOSIM 10IU :
The dose of these medicines will be different for different patients. Follow your doctor’s orders or the directions on the label . The following information includes only the average doses of these medicines. If your dose is different, do not change it unless your doctor tells you to do so.
For treatment of growth failure caused by growth hormone deficiency:Adults: Dose is based on body weight and must be determined by your doctor. At first, it is usually 0.005 milligram (mg) per kilogram (kg) (0.0023 mg per pound) of body weight injected under the skin once a day. Your doctor may then increase the dose if needed.Children: Dose is based on body weight and must be determined by your doctor. The usual total weekly dose is 0.16 to 0.3 mg per kg (0.073 to 0.136 mg per pound) of body weight. This is divided into smaller doses and usually is injected under the skin, but may be injected into a muscle as determined by your doctor.
For treatment of growth failure caused by kidney disease:Children: Dose is based on body weight and must be determined by your doctor. The usual total weekly dose is 0.35 mg per kg (0.16 mg per pound) of body weight. This is divided into smaller daily doses and is injected under the skin or into a muscle.
For treatment of growth failure caused by Turner’s syndrome:Children: Dose is based on body weight and must be determined by your doctor. The usual total weekly dose is 0.375 mg per kg (0.17 mg per pound) of body weight. This is divided into smaller doses and is injected under the skin.
For treatment of growth failure caused by Prader-Willi syndrome:
Children: Dose is based on body weight and must be determined by your doctor. The usual total weekly dose is 0.24 mg per kg (0.11 mg per pound) of body weight. This is divided into 6 or 7 smaller doses over the course of the week and is injected under the skin.
For treatment of weight loss caused by acquired immunodeficiency disease (AIDS):
Adults weighing more than 121 pounds (55 kg)—6 mg injected under the skin once a day at bedtime.
Adults weighing 99 to 121 pounds (45 to 55 kg)—5 mg injected under the skin once a day at bedtime.
Adults weighing 77 to 98 pounds (35 to 44 kg)—4 mg injected under the skin once a day at bedtime.
Adults weighing less than 77 pounds (35 kg)—Dose is based on body weight and must be determined by your doctor. It is usually 0.1 mg per kg (0.045 mg per pound) of body weight injected under the skin once a day at bedtime.
Children: Use and dose must be determined by your doctor.
Warnings and precautions:
In adult women on oral estrogen replacement, a larger dose of somatropin may be required to achieve the defined treatment goal (see DOSAGE AND ADMINISTRATION).
In patients with diabetes mellitus requiring drug therapy, the dose of insulin and/or oral agent may require adjustment when somatropin therapy is initiated
In adult women on oral estrogen replacement, a larger dose of somatropin may be required to achieve the defined treatment goal (see DOSAGE AND ADMINISTRATION).
In patients with diabetes mellitus requiring drug therapy, the dose of insulin and/or oral agent may require adjustment when somatropin therapy is initiated
See CONTRAINDICATIONS for information on increased mortality in patients with acute critical illness due to complications following open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure. The safety of continuing somatropin treatment in patients receiving replacement doses for approved indications who concurrently develop these illnesses has not been established. Therefore, the potential benefit of treatment continuation with somatropin in patients having acute critical illnesses should be weighed against the potential risk.